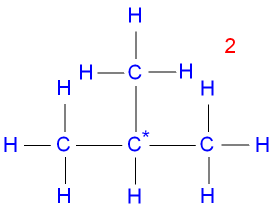
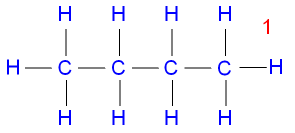
They have different structural formulas, hence different physical properties for example different melting and boiling points.
Isomerism in alkanes must start with molecules that have more than 3 carbon atoms, so methane, ethane and propane do not have isomers.
General formula=CnH2n+1
Isomerism in Butane:
No comments:
Post a Comment